TOPICAL FORM OF RADICALS SCAVENGER
Project Summary
CVXL-0095 Project proposes an innovative formulation, allowing the local delivery of a molecule with proven anti-radical properties to healthy tissues through which ionizing radiation passes, to:
- Reduce the severity and duration of oral mucositis induced by radiotherapy and chemotherapy in the management of ENT cancers,
- Reduce the occurrence and duration of radiation-induced dermatitis and chemotherapy in the management of breast cancer,
- Treat side effects (local mucositis) related to radiotherapy in the management of other cancers such as ovarian or prostate cancer, among others.
CPh-1014, an active metabolite of amifostine formulated as a thermosensitive gel, has demonstrated its efficacy in reducing the severity of oral or skin lesions induced by ionizing radiation in established preclinical models of oral mucositis or radiodermatitis.
The Scientific advisor of European Medicines Agency (EMA) has agreed to the preclinical development plan and once the local tolerance has been studied, the therapeutic efficacy of CPh-1014 can be established in an extended and “pivotal” Phase II trial.
Background and therapeutic objectives
Dermatitis or mucositis is one of the adverse effects of the treatment of ENT, digestive and urogenital system or breast cancers. Our innovative formulation allows the effective delivery, at the local level, of an antiradical agent that protects cells from the aggression of radiation or chemo-therapeutic agents. The concept of the beneficial effect of antiradical molecules in the treatment of mucositis induced by radiotherapy has been established in clinical studies, but with molecules administered intravenously.
Radiation or chemo-therapeutic agents induce oxidative stress in the cells, causing the accumulation of free radicals and damage to DNA. DNA damage triggers a pro-inflammatory response in the tissue. This local response results in lesions that often lead to ulceration with an increased risk of bacterial infection.
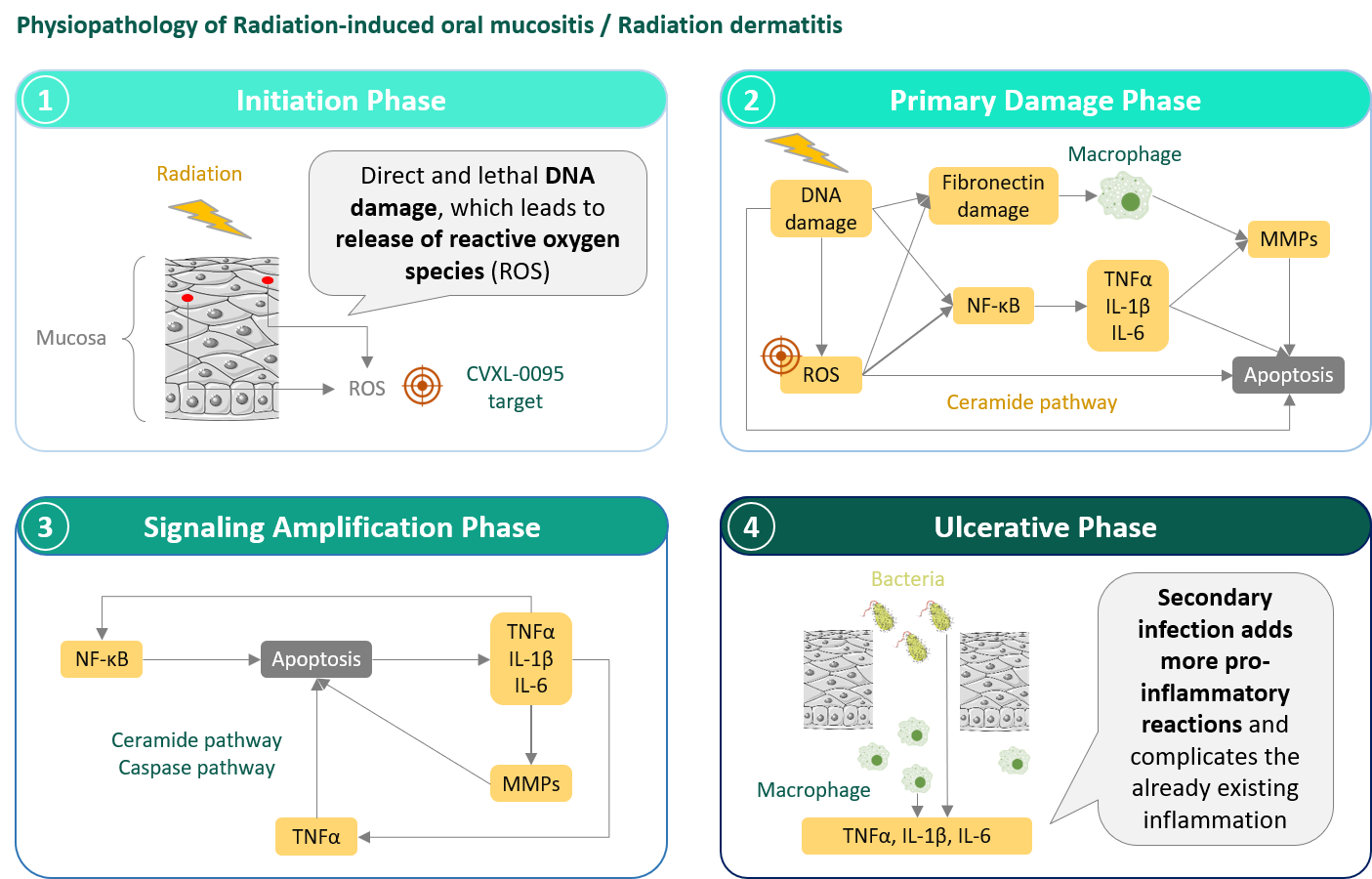
The initial discomfort then, as the lesions progress, the pain felt by the patient often requiring the use of morphine, the inability to ingest solid food in the case of ENT cancers, generally ceases only after the treatment is stopped, the time to allow the healing of the damaged tissues. As a result, the entire anti-cancer strategy is put at risk.
By tackling this phenomenon from its initiation phase, CPh-1014 limits the frequency, duration and severity of its occurrence. Unlike competitors administered intravenously, therefore under medical supervision, our formulation is designed to be easy to apply by the patient himself and to be integrated, without disturbing the patient, into the treatment cycle of their cancer in Oncology centers.
